The Alcudia archaeological site correspond to the ancient city of Ilici (Roman Colonia Iulia Ilici Augusta). The site consists of an succession of Iberian, roman, byzantine, Visigoth and Moorish settlements (since the 5th centuries BC to the 8th century AC) on the top of a hill that in the 1st century BC was surrounded by a wall and nearby the Vinalopó river mouth in the Bajo Segura Bay. This bay was known in Roman times as the sinus Ilicitanus. Different clays of the area nearby to the site have been sampled, analysed and then compared with analysis of some pottery remains of the different periods of the Alcudia settlement. The clays have been analysed by micro-X Ray fluorescence (µXRF) and by infrared spectrometry by Fourier transformed (ATR-FTIR), as crude, mixed in different proportions, and then heated to a different temperature until 900ºC. The old remains show affinities with the clays sampled from the Eastern part indicating a W-E influence, as the northern coast of the sinus ilicitanus. During the roman period and later the pottery was made using the nearby clays located just to the north of the site clearly influenced by the via Augusta. The topography to the north of the site is formed by low but steep hills and small creeks that possibly limit the commerce to the north, so the commerce follows the marine plain coast of the northern sinus ilicitanus. The opening of the roman road via August along the Vinalopó Valley favoured the use of pottery clays from the interior of the territory.
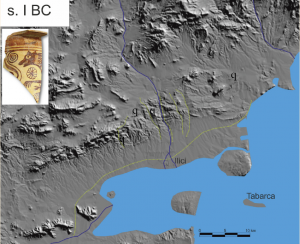
Paleogeographic map of the area north of the sinus ilicitanus. The clays used for pottery were obtained from the area parallel to the north shore of the sinus. Pottery: Variable carbonate no sílices
Cite as: Jordá Guijarro, J., Cerdán Sala, M., Sánchez-Sánchez, A., Ronda Femenia A., Tendero Porras, M. and Tent-Manclús, J. E. (2021): Pottery clays quarries in the domain territory of ancient Ilici (Alicante, SE Spain). LAC1+1, Landscape archaeology conference, 8-11 june 2021 Book of Abstracts. 45 p.
Tags: Elche, Holocene, illice, pottery clay, sinus ilicitanus, via augusta
Change on the coastline of the Southern Alicante region
Tent-Manclús, J. E.
The Bajo Segura Region is a plain dominated by the Segura River with the mouth towards the Mediterranean Sea on Guardamar. The Hondón and Salinas de Santa Pola lagoons inside the plain, are the remains of a major marine bay known in the Roman times as the sinus ilicitanus. Offshore the Region geophysical data has permitted to model the coastline change and study the underwater marine terraces. 183 maps have been made from the 15000 BP until Today (100 year apart the older ones and 25 years for the recent ones). The coastline has migrated between 20 and 50 km onshore and then offshore about tens of meters to 35 km shaping today coastline. The sea level at 15000 BP was located 70 m below today sea-level (btsl). During the Younger Dryas (13500 to 11700 BP) varies around 60-66 m btsl then increase until 21 m btsl and stopped during the 8.2 ka event (8500 BP to 8200 BP). Again, the level increases first fast and then slowly but moving fast horizontally (near 35 km for 1000 years) developing the sinus ilicitanus when the marine transgression enters the plain about 4200 BC. The Segura River developed a delta in the today area of Orihuela, 35 km to the East of its today river mouth in Guardamar. The river until around the year 1400 AD developed a fingering (bird-foot) quite variable delta in the center of the sinus, most of the major irrigation canals (acequias) could correspond to the ancient delta-channels. Around the XV century major events changed the sinus basin producing the river mouth move to the present location. The sinus open communication with the Mediterranean Sea was closed around the XVIII century by sand bars, first the southern of Guardamar and then the northern on Santa Pola.
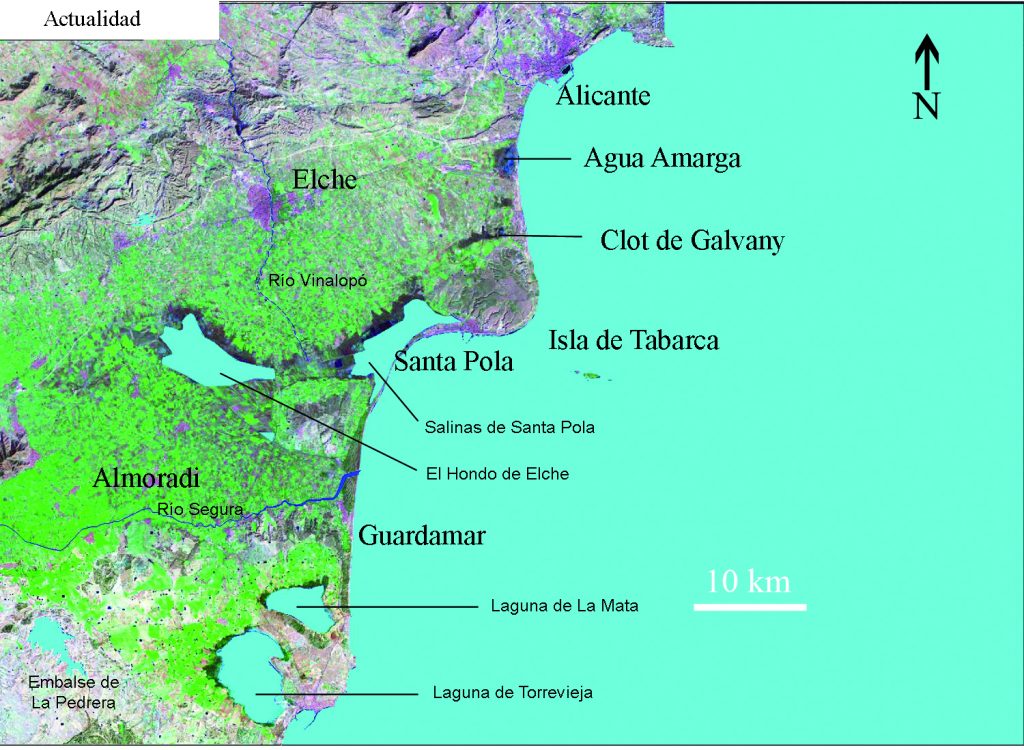
Cite as:
Tags: Alicante coast, coasline change, Holocene, sea-level change, Segura River, sinus ilicitanus
This year the students of the third course of Marine sciences of the Alicante University, could not go for a field trip on November as previous years, visiting the Sorbas basin, Cabo de Gata, and Hoyazo de Nijar. When the courses ended on May most of the students did the visit. The visit took place on 27 and 28 of May of 2021.
The field trip was strange because of the new normality and also the rain comes to Almeria during the visit.

Alicante University students of Marine Sciences in the Sorbas basin
The professors in charge were Juana Jordá, and José Enrique Tent-Manclús.
Tags: Holocene
Students of the third course of Geology of the Alicante University within the subject of Regional Geology: visit La Albufera de Valencia. First we have a view of the Ermita dels Sants de la Pedra (Sueca) and then the Silla Port. The evolution of the Albufera from the Middle Ages when was connected with the sea to the today lake was explained. The Moorish expulsion using the today disappeared Sueca Port was pointed as the one of the subject to work with.

Students watching the smoke produced by the rice fields fires
Tags: Albufera, Alicante University, Holocene
The change of the coastline in the southern part of the province of Alicante for the last 15,000 years have been modeled and described in the work of Tent-Manclus (2013). It is obtained by integrating data from different sources and especially high-resolution seismic profiles of the nearby marine continental shelf. Ten periods have been distinguished in the evolution of the Bajo Segura coastline : 1) 15000 BP- 14600 BP: rapid rise; 2) 14600 BP to 13500 BP: the Older Dryas, slow rise; 3) 13500 BP to 12700 BP: stability and descent; 4) 12700 BP to 11700 BP: the Younger Dryas, descent of the sea level; 5) 11700 BP to 11300 BP: very fast rise; 6) 11300 BP a 8500 BP: rapid rise; 7) 8500 BP = 6500 AC to 8200 BP = 6200 AC: 8.2 ka event stabilization; 8) 6,200 BC to 4,000 BC: rise, Tabarca Island is form; 9) 4000 BC to 3000 BC: the slow rise due to subsidence sinus ilicitanus forms and 10) 3000 BC to the present day 2000 AD: the sinus ilicitanus desiccates.

Map of the southern Alicante coast area used in the work
Maps at different times are show selected from the 164 maps that have been made. This is a first version of how the coastline evolves in the region. This work It can be a starting point to improve knowledge of the historical landscape evolution..
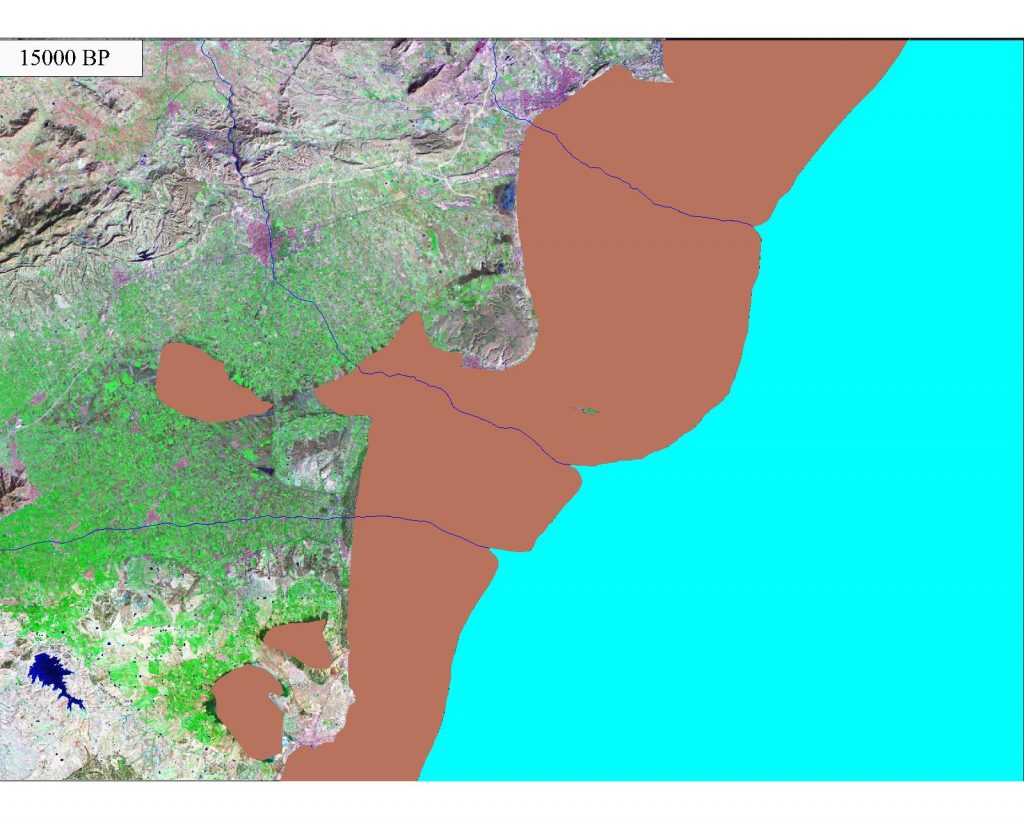
Map of the South Alicante coast 15000 BP. The sea level was 70 m below today level. The rivers Vinalopo and Segura were running parallel.
The sinus ilicitanus dries until today, the rest of which are the lagoons of the Fondo and Santa Polo Salinas.
Cite as: Tent-Manclús, J. E. (2013): Cambios de la línea de costa en el Bajo Segura (S de Alicante) en los últimos 15.000 años. Estudios geográficos, 74 (275): 684-702. Doi: 10.3989/estgeogr.201324
Tags: Alicante coast, Bajo Segura, coastal maps, Holocene, sea-level change
Last February 6, 2020, students in the third year of the Marine Geology Degree carried out practices aboard the “Guadalupe” boat. This 12m-length boat assigned to the City of Dénia by the Court of Instruction number 3 of Dénia by the Judge Javier Reyes, is dedicated not only to surveillance tasks of the Marine Reserve of the Cape of San Antonio, but also to operations of pollution control and scientific monitoring of the protected area.
The practice was focused on different aspects of “Geophysics and geophysical prospecting”, under the supervision of Professor José Enrique Tent Manclús. Within a research program of the Multidisciplinary Institute for the Study of the Environment “Ramón Margalef” (IMEM) of the University of Alicante that together with the City Council of Dénia and the National Parks Autonomous Organization operate the Montgó-Dénia Scientific Station (ESCIMO-Dénia).
Taking advantage the good conditions at sea, marine geophysics equipment was tested, specifically a high-resolution marine boomer equipment linked to a differential GPS. The practice was to leave the port, perform the boot sequence and navigate acquiring data. Through sound waves, this equipment allows interpreting the geometry of the layers below the seabed using the same principle as the medical ultrasound, which was precisely developed from this marine technology. Like 2d ultrasound, experience and practice are required for interpretation.
In this first marine campaign, it has been tested the equipment adjustment settings to obtain a better results. The students have been able to see in situ how the equipment studied in the classroom works and, above all, have a first contact with work in the marine environment, much more hostile than the terrestrial to carry out any study.

Student inside the Guadalupe boat during the sailing.
The marine area of the San Antonio Cape is the extension of the rocky massif of Montgó. The equipment is intended to see the thicknesses of the sandbars and / or sediments trapped by the Posidonia oceanica, above the rocky bottoms. In a future campaign will be locate and delimit the bodies of sand and how they change to areas without sand. In the deepest parts it is expected to be able to locate some level of marine terrace formed when the sea level was lower as a result of the last glaciation. At the last glacial maximum, 18,000 years ago, the sea level was 120 m below the current level. A warming began that caused a rise of 1 to 2 cm a year, but about 14,000 and 11,700 years ago the global climate cooled, which stopped the ascent. This generated rocky coasts that are currently marine terraces between 70 and 60 m deep. After this cold interval known as the “Younger Dryas”, the level rose to reach its current position about 6000 years ago. Younger Dryas terraces have already been located in the coastal area Tabarca Island (South coast Alicante) and in the near future it is intended to study these terraces in the area of San Antonio Cabe.
This study of marine geology together with others of a biological and physical-chemical nature, promoted by the Montgó Scientific Station (ESCIMO-Dénia), not only seeks to improve the knowledge of a very interesting area at the geological level but also to lay the foundations for a proposal to expand and improve the fishing resources for the artisanal fleet around the Marine Reserve as well as the promotion of sustainable activities such as diving.
Tags: Denia, ESCIMO-Denia, Holocene, IMEM, University Alicante
As the last year, students of the third course of Marine sciences of the Alicante University visit the Sorbas basin, Cabo de Gata, Hoyazo de Nijar, and Cerro del Espíritu Santo of Vera. The visit took place on 7 and 8 of November of 2019.
The visit was organized by Antonio Estévez, Santiago Moliner, and José Enrique Tent-Manclús.

Student group before entering the Sorbas Cave.

The logo was made by Jesús M. Soria based in an eroded mediterranean margin resembling the M of Messinian.
The “Paleoenviromental changes” logo is for the Research Group of the Alicante University.
The Alicante Messinian Group was granted by the Ministerio de Ciencia e Innovación Research Project CGL 2007 -6583 .
Tags: projects
Diego García-Ramos
University of Vienna, Institut for Paläontologie
1.Introduction
Brachiopods, a name that derives from Ancient Greek words βραχίων (“arm”) and πούς (“foot”), make up a phylum of tripoblastic, coelomate, marine invertebrates that belong, along with bryozoans and phoronideans, to the superphylum of the so-called lophophorates. Their body plan consists of two valves displaying bilateral symmetry, and are generally inequivalve. Most brachiopods are sessile, stenohaline suspension feeders, and their fossil record spans from Lower Cambrian times to Recent. Today’s brachiopod diversity shows that they constitute a relic group of animals when compared with their paleobiodiversity during past geological time, especially in the Paleozoic, throughout which brachiopods were extremely diverse and abundant. Nowadays there are some 391 extant species of which around 5 % may be synonyms (Emig et al., 2013). Extant brachiopods are distributed from the intertidal zone to abyssal depths, but they occur more frequently in the inner and outer shelf, and the epibathyal zone.
- Brachiopods as paleocological and paleobiogeographical proxies
Since brachiopods are overall facies dependent organisms (Ager, 1965), together with the fact that many of their species display a long-ranging vertical distribution, their use as guide-fossils is limited in comparison with other groups like ammonoids and calcareous micro- and nannoplankton, despite the fact that many Paleozoic brachiopods are important biostratigraphical markers. In any case, the precise feature that they are facies-linked animals make brachiopods considerably suitable for paleoecological and paleogeographical studies. In particular, Ager (1967) outlined a number of reasons why fossil brachiopods may be of great use in paleoecological studies: 1) their great abundance throughout the Phanerozoic; 2) their occurrence in diverse sedimentary facies; 3) their susceptibility to environmental differences and perturbation; 4) the fact that their skeletal traits record physiological differences which are related to their life habit; and 5) the survival, with a relatively adequate diversity, of modern representatives which may aid in actuopaleontological analyses.
As for their use in paleobiogeography, Smirnova (2012) stressed the fact that brachiopods, most of which were/are fundamentally shelf dwellers, are good proxies to estimate the proximity of the coastline, and enable to contour marine basins accurately. Other than that, she also pointed out that brachiopods are sensitive to climatic factors, and the taxonomic composition of brachiopods has been proven to be a good tool in differentiating paleobiogeographical realms.
- Overview of Tertiary brachiopods from the Betic Cordillera
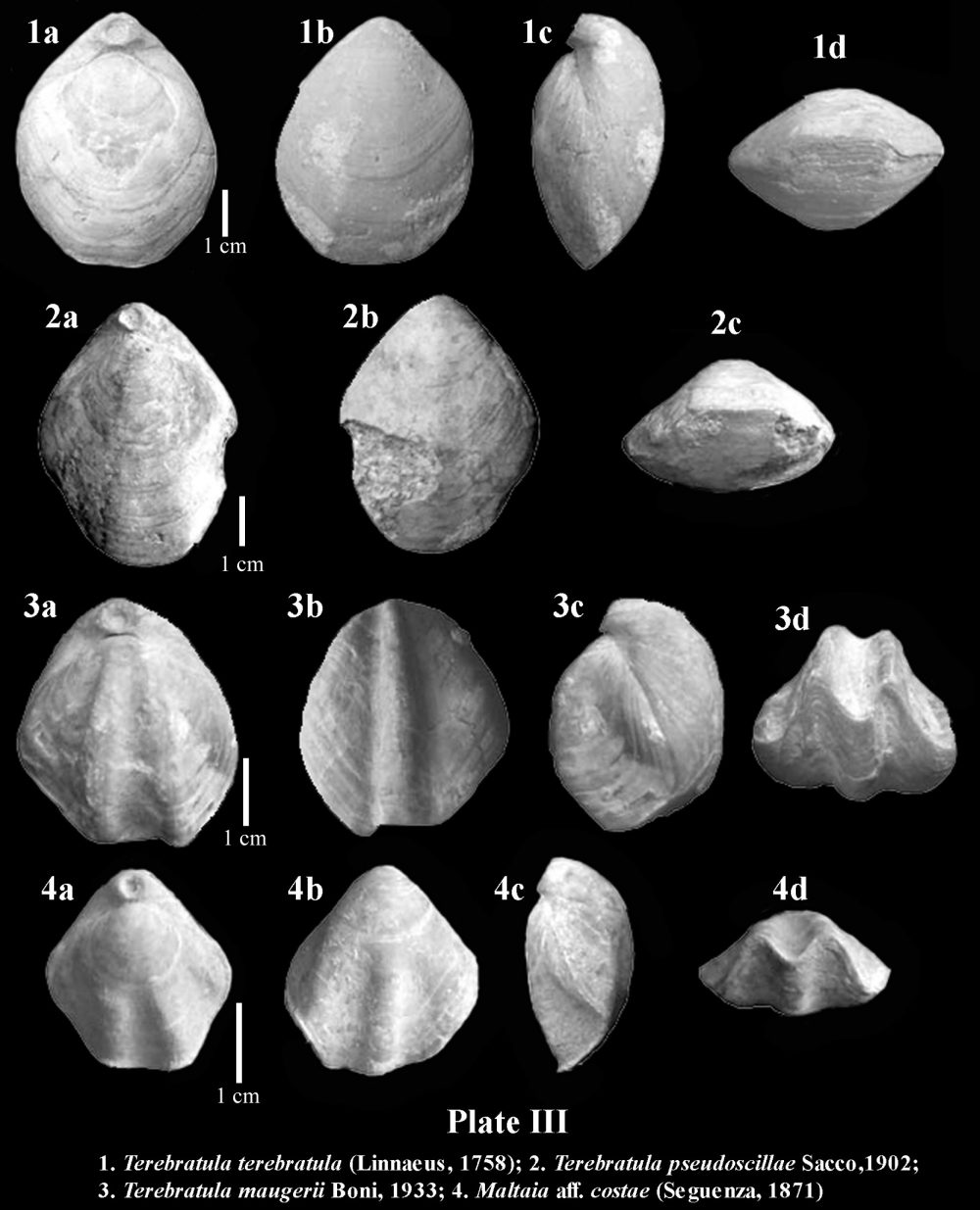
Brachiopods in the Tertiary record of the Betic Cordillera are relatively poorly known. There are sparse citations in old publications such as that of Davidson (1864), who mentioned the occurrence of Megerlia truncata(Linnaeus, 1767) in the Miocene of Gibraltar, or Terebratula sinuosa (Brocchi, 1814) in Miocene rocks from Córdoba (Davidson, 1870) (later known to be Terebratula maugerii Boni, 1933). Kilian in Fouqué (1884) cited a number of species of Terebratula, besides the rhynchonellid and thecideid brachiopods Aphelesia bipartita(Brocchi, 1814) and Lacazella mediterranea (Risso, 1826), from the Neogene record of the Granada Basin.
A general glance at Cainozoic (but mainly Neogene) brachiopod paleobiodiversity in the Betic Cordillera shows that the genus Terebratula Müller, 1776 prevails by far, making up more than 80% of the record of brachiopods during this era (Toscano et al., 2010). In rocks of Eocene age, terebratulids attributable to Carneithyris hilarionis (Davidson, 1870) are known to occur rarely in the province of Alicante (Sulser et al., 2010), and there is unpublished record of Upper Oligocene to Lower Miocene small gryphine brachiopods, along with Terebratulina gr. tenuistriata (Leymerie, 1846), in the province of Murcia.
Two main types of brachiopod assemblages may be recognized in Neogene deposits of the eastern Betic Cordillera: a relatively shallow water Terebratula–Aphelesia assemblage associated to fine-grained bottoms, forming Terebratulapavements or clumps, with sparse, solitary occurrence of Aphelesia, and a bathyal assemblage where small gryphine-like terebratulids and Ceramisia meneghiniana (Seguenza, 1865) are distinctive components, found in deep-sea hard-substrates and associated to bamboo-coral bioherms (see Barrier et al., 1991). Other taxa such as Megerlia truncata, Terebratulina retusa (Linnaeus, 1758) and Lacazella mediterranea display an euribathic distribution and may occur in both assemblages as long as there is suitable substrate. The micromorphic brachiopod Megathiris detruncata (Gmelin, 1790) and the inarticulate Ancistrocrania abnormis(Defrance in Hoeninghaus, 1828) have been found in infralittoral to circalittoral hard-substrates consisting either of shelly-grounds or rocky-grounds. Joania cordata (Risso, 1826) and Novocrania turbinata (Poli, 1795) have exclusively been found in hard-substrates corresponding to a circalittoral volcanic swell.
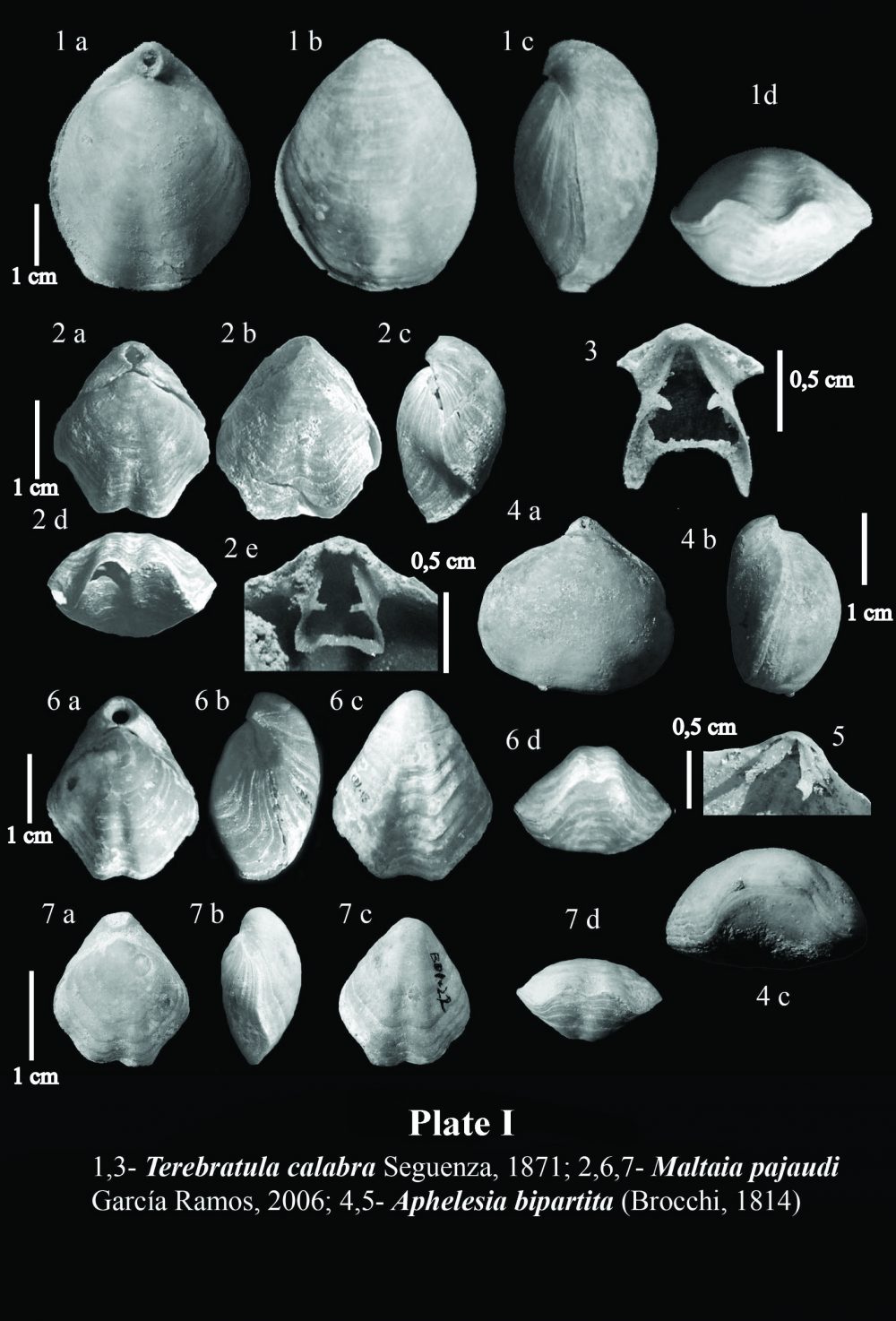
In sediments of Late Neogene age deposited the intramountaneous basins of the Betic Cordillera, the recurrent assemblage of species of the genera Terebratula -or its close relative Maltaia Cooper, 1983-and Aphelesia Cooper, 1959, has been already pointed out elsewhere by, for instance, Pedley (1976) and Gaetani (1986). During the Tortonian, the species Aphelesia margineplicata(Philippi, 1844) commonly co-occurred in silty to fine-grained sandy lithofacies in association with Terebratula maugeriior Terebratula pseudoscillae Sacco, 1902 (see also Calzada, 1978). In addition, it has also been found in the same outcrops together with Miocene representatives of Terebratula calabra Seguenza, 1871, or Maltaia aff. costae (Seguenza, 1871), in Late Tortonian to Early Messinian aged deposits, mainly interpretable as of infralittoral to circalittoral paleoenvironments.
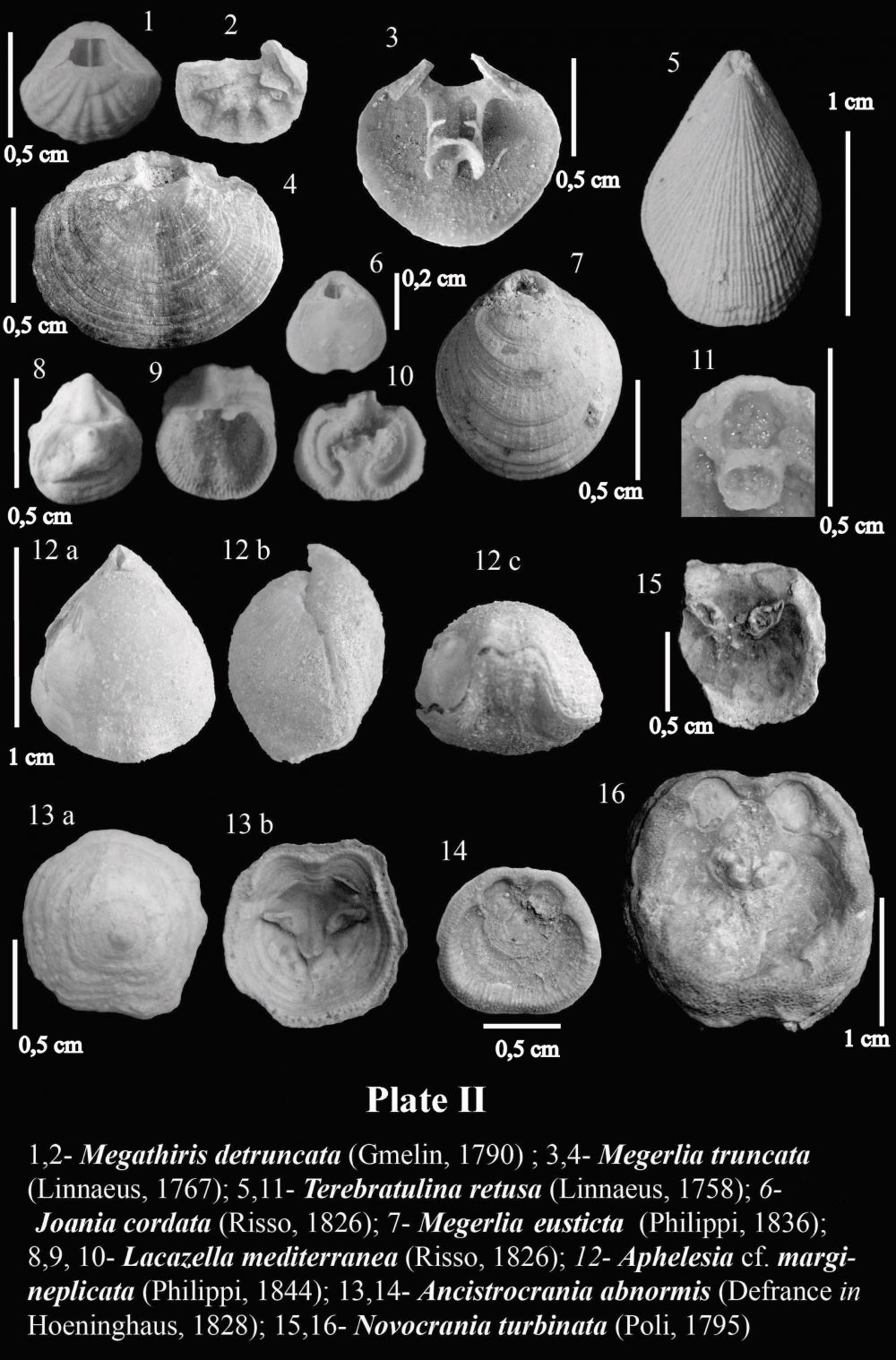
The onset of the Messinian Salinity Crisis exerted a profound impact on practically the whole marine paleobiota of the Mediterranean at the end of Messinian times, and brachiopods were not an exception, but they were among the victims of this vast extinction. The restoration of normal marine conditions that followed the opening of the Strait of Gibraltar, leading to the Early Pliocene re-flooding, implied a re-colonization by brachiopods, ever since Terebratula and Aphelesia had survived in Atlantic waters (see, for example, Dollfus & Cotter, 1909 and Toscano et al., 2010). The taxonomic composition of Pliocene Terebratula–Aphelesiaassemblages of the Betic Cordillera marked a faunal turnover when compared to those prevailing during the Miocene. The species Aphelesia bipartita replaced A. margineplicata –which presence became marginal- as the dominating partner of Terebratula (e.g. Pajaud, 1976). Likewise, T.pseudoscillae and the once abundant Terebratula maugerii= T. sinuosadied out. Their niche was taken over by Terebratula terebratula (Linnaeus, 1758), by Pliocene morphotypes of Terebratula calabra, and by Maltaia pajaudi García-Ramos, 2006.
4.Brachiopods and Betic Corridors
Reolid et al. (2012) have shown that the genus Terebratula might have likely thriven in the shelf margins of Atlantic-Mediterranean corridors by benefitting from warmer and more saline water currents flowing from the Mediterranean to the Atlantic. The occurrence of Terebratula in Betic intramountane basins that might perform as Atlantic-Mediterranean seaways can be traced as far back as to Early Miocene times (e.g. García-Ramos, 2006). Llarena (1934) mentioned the presence of Terebratula sp. and Terebratula grandis (probably T. pseudoscillae) in basins located within the Prebetic realm of the province of Albacete, and Darder Pericás (1945) likewise described outcrops yielding numerous specimens of Terebratula in Neogene basins of the Prebetic in the southern sector of the province of Valencia. All of these basins might be part of an interconnected web of Atlantic-Mediterranean seaways. Further studies are needed to solve the puzzle as to how the Atlantic-Mediterranean connections evolved in the Betic Cordillera as the Alborán Block was undergoing indentation against the Iberian paleomargin. The study of marine epibenthic macroinvertebrates, including brachiopods, may play a role in enhancing our knowledge of how all of this happened.
References:
Ager, D.V., 1965, The adaptation of brachiopods to different environments: Palaeogeography, Palaeoclimatology, Palaeoecology, 1, p. 143-172.
Ager, D.V., 1967, Brachiopod palaeoecology: Earth-Science Review, 2, p. 157-159.
Barrier, P., Zibrowius, H., Lozouet, P., Montenat, C., Ott d’Estevou, P., Serrano, F., Soudet, H. J., 1991, Une faune de fond dur du bathyal superieur dans le Miocene terminal des Cordilleres Betiques (Carboneras, SE Espagne): Mesogee (Bulletin du Museum d’Histoire Naturelle de Marseille), 51, p. 3-13.
Bertrand, M., Kilian, W., 1884, Études sur les terrains secondaires et tertiaires dans les provinces de Grenade et de Malaga, in : «Mission d’Andalousie » : études relatives au tremblement de terre du 25 décembre 1884 et à la constitution géologique du sol ébranlé par les secousses: Mém. Acad. Sci. Paris, 30 (2), p. 377–579.
Calzada B. S., 1978, Braquiópodos tortonienses de Murcia: Estudios geológicos, 34, p.351-358.
Davidson, T., 1864, Description of the Brachiopoda: in Adams S L. (ed.), Outline of the geology of the Maltese Islands. The Annals and Magazine of Natural History, Series 3, 14, p. 5-11.
Davidson, T., 1870, On Italian Tertiary Brachiopoda: Geological Magazine, London, 7 (8), p. 359-370, p. 399-408, p. 460-466.
Darder Pericás, B., 1945, Estudio geológico del sur de la Provincia de Valencia y norte de la de Alicante. Boletín Geológico y Minero de España, 57: p. 59-837.
Dollfus, G.F. & Cotter, J.C.B, 1909, Le Pliocène au Nord du Tage (Plaisancien).Brachiopoda. Commission du service géologique du Portugal, p. 87-90.
Emig, C. C., Bitner, M.A., Álvarez, F., 2013, Phylum Brachiopoda. In: Zhang, Z.-Q. (Ed.) Animal Biodiversity: An Outline of Higher-level Classification and Survey of Taxonomic Richness (Addenda 2013). Zootaxa, 3703, 1–82.
Gaetani, M., 1986, Brachiopod paleocommunities from the Plio-Pleistocene of Calabria and Sicilia (Italy). In: Biostratigraphie du Paléozoïque 4. Les brachiopodes fossils et actueles. P.R. Racheboeuf & Emig, C.C. (Ed.). p. 281-288.
García Ramos, D.A. 2006. Nota sobre Terebratulinae del Terciario de Europa y su relación con los representantes neógenos del sureste español. Boletín de la Asociación Cultural Paleontológica Murciana 5: 23-83.
Llarena, J.G. de, 1934, Observaciones sobre la geología y fisiografía de los alrededores de Hellín. Boletín de la Real Sociedad Española de Historia Natural, p.213-231.
Pajaud, D., 1976, Les Brachiopodes du Pliocène I de la Sierra de Santa Pola (sud d’Alicante, Espagne): Terebratula terebratula (Linné, 1758) et Phapsirhynchia sanctapaulensis nov. gen., nov. sp.: Annales de la Société géologique du Nord, 96, p. 99-106.
Pedley H. M., 1976, A palaeoecological study of the Upper Coralline Limestone, Terebratula-Aphelesia Bed (Miocene, Malta) based on bryozoan growth-form studies and brachiopod distributions: Palaeogeography Palaeoclimatology Palaeoecology, 20, p. 209-234.
Smirnova, T.N., 2012, Early Cretaceous Rhynchonellids of Dagestan: System, Morphology, Stratigraphic and Paleobiogeographic Significance. Paleontological Journal, v.46, nº 11, p. 1197-1296.
Reolid, M., García-García, F., Tomasovych, A. and Soria, J.M., 2012, Thick brachiopod shell concentrations from prodelta and siliciclastic ramp in a Tortonian Atlantic–Mediterranean strait (Miocene, Guadix Basin, southern Spain). Facies 58: 549-571.
Sulser, H., García-Ramos, D., Küsteiner, P., Menkveld-Feller, U., 2010, Taxonomy and palaeoecology of brachiopods from the South-Helvetic zone of the Fäneren region (Lutetian, Eocene, NE Switzerland). Swiss Journal of Geosciences, 103: p. 257–272.
Toscano-Grande, A., García-Ramos, D., Ruiz-Muñoz, F., González-Regalado, M.L., Abad, M., Civis-Llovera, J., González-Delgado, J.A., Rico-García, A., Martínez-Chacón, M.L., García, E.X. and Pendón-Martín, J.G. 2010. Braquiópodos neógenos del suroeste de la depresión del Guadalquivir (sur de España). Revista Mexicana de Ciencias Geológicas 27: 254-263.
This post is an extended abstract of the work by Diego García-Ramos (2006): Nota sobre terebatulinae del Terciario de Europa y su relación con los representantes neógenos del sureste español. Boletín de la Asociación Cultural Paleontológica Murciana. 5, pp. 23-83
Tags: Betic cordillera, Braquiopods

Recent Comments